Chaetoceros ceratosporum, in deep - sea water pumped from Toyama Bay.
by Keiichi Shozen1, Itaru Umeda2, Toshimitu Nakashima 3 Akio Honma4
1Toyama Prefectural Fisheries Research Institute364 Takatsuka, Namerikawa, Toyama, 936-8536, Japan"
2Ebara Corporation, 1-6-27, Konan, Minato, Tokyo, 108-8480, Japan
3Japan Marine Science and Technology Center, 2-15, Natsushima, Yokosuka, Kanagawa, 235-0061, Japan
4Japan Sea-Farming Association, 3-14-8, Uchikanda, Chiyoda, Tokyo, 101-0041, Japan
ABSTRACT
In order to establish long-term continuous culture of a planktonic diatom, experiments using Deep-sea water (DSW) as the culture medium for Chaetoceros ceratosporum OSTENFELD were carried out. DSW is nutrient-rich compared with surface sea water. We used a chemostat system for phytoplankton, Ebara Photo-bio-reactor (PBR-60, Ebara Co. Ltd.). Water temperature and pH were adjusted to 17±1¢J and 7.8±0.1, respectively. Culture medium was stirred at 50 rpm and aerated at a rate of 60L air/min. Illumination was provided by halogen lamps with a photo flux density between 90 to 120 £g E/m2S for 24 hours. In continuous culture, the turnover rate of DSW was elevated step by step from 0.2 to 1.2/day. The cell density fluctuated from 10¡Ñ104 to 61¡Ñ104cells/ml in continuous culture; high level of cell density was obtained for as long as 125 days. Since there was no difference in yield of cells between turnover rates 1.0 and 1.2/day, 1.2/day became the upper limit state. N-nitrate and P-phosphate were almost consumed at each turnover rate in continuos culture. The consumption rate of silicate was lower than nitrate and phosphate, and decreased with turnover rate. However, more than 80% of silicate was consumed. The nutrients of DSW were consumed efficiently in the continuous culture device.
INTRODUCTION
In Toyama Prefecture Fisheries Research institute, 3000 tons per day of deep-sea water (DSW) have been pumped from a depth of 321m in Toyama Bay (Nakamura 1999). DSW, as compared with surface-sea water, has low temperature and biological cleanliness. The cold, clean DSW is mainly used for stock enhancement or ecological studies of cold water/deep-sea species of fish and shellfish in our Institute (Matsuzato 1998, Nakamura 1999). Another characteristic of DSW, nutrient-richness, has been also studies in microalgal culture (Matubayashi et al. 1994). Diatoms, in previous trails using DSW, were batch cultured in flasks (Nakashima 1988, 1992 a, 1992 b, Fukami 1992) by small-scale or short-term continuous culture, for several days or dozens of days (Fukami et al. 1998).
Our experiment was carried out to establish long-term continuous culture of a planktonic diatom, Ch. ceratosporum OSTENFELD, using DSW as the sole source of nutrients. This is a well-known species, which has been used as a diet for brine shrimp, bivalves, planktonic larvae of sea urchins, and rock oyster larvae (Takano 1967, Tanaka 1982, Tenjin & Ishii 1984). Yield of this diatom may support a new project in the multiple/cascade use of deep-sea water.
MATERIAL AND METHODS
Strain
Chaetoceros ceratosporum OSTENFELD (Fig.1) isolated from Sagami Bay (Tanaka 1982) was kindly provided by the Japan Marine Science and Technology Center. We cultured this strain at 15 ¢J with a photo flux density of 40 E/m2S for 24
hours for this experiment.

Deep-sea water
Water temperature of DSW varied between 2- 4 ¢J and was 2.3 ¢J on average. Salinity of DSW remained around 34.1 before use. Concentration of nutrients, N-nitrate, P-phosphate and Si-silicate were 5.92 £g M, 1.55 £gM and 35.60 £g M, respectively.
¡@
To determine the temperature of continuous cultivation, batch culture was carried out for 14 days using 200 ml flasks filled with 100ml DSW at four temperatures--10, 15, 20 and 25 ¢J --and cell density was checked every day. Each flask was aerated at a rate of 100ml/min. Illumination was provided by white fluorescent tubes, giving a photo flux density of 80 £g E/m2S for 24 hours. At the beginning, 5,000 cells/ml of Ch. Ceratosporum was inoculated in each flask. Culture growth was measured by counting cell numbers with a hemacytometer.
¡@
Continuous cultivation
We used an Ebara Photo-bio-reactor (PBR-60, Ebara Co. Ltd.) which is device for phytoplankton, and cultured Ch. ceratosporum with a chemostat system in which the supply of culture medium and collection of phytoplankton yield are conducted by one roller pump (Fig. 2). The volume of the culture tank is 180 L. The pumped deep-sea water, as the culture medium, is clean; two sediment tanks were placed before pumping to minimize contamination and no filtration was used. Illumination was provided by 6 built-in halogen lamps with a photo flux density between 90 to 120 £g E/m2S for 24 hours. For water temperature control, 15 degree-C was targeted but the temperature increased up to 17±1 ¢J due to heat emission from the halogen lamps. pH was adjusted to 7.8±0.1 using carbon dioxide since the pH of deep sea water is around 7.8. Culture medium was stirred at 50 rpm and aerated at a rate of 60L/min. Cultivation was initiated under batch conditions and shifted to continuous stage by adding DSW after cell density reached 10¡Ñ104 cells/ml. The turnover rate of DSW was elevated from 0.2/day to 1.2/day gradually during continuous cultivation.
RESULTS AND DISCUSSION
Preliminary Batch cultivation
The growth curve of Ch. Ceratosporum at each batch culture is shown in Fig. 1. At 10, cell density increased slowly and peaked at 3.6¡Ñ104 cells/ml in 14 days. At 15¢J, cell density reached a peak of 14¡Ñ104 cells/ml in 7 days and then it fluctuated from 9¡Ñ104to 14¡Ñ104 cells/ml. At 20 and 25¢J, cell density reached 14¡Ñ104 and 17¡Ñ104 in 3 days, respectively, but after 6 days density decreased gradually and reached about 2¡Ñ104 cells/ml in 14 days. As the result, we selected 15 ¢J as the water temperature of continuous culture since the maximum yield was not so far from those of higher temperatures. Considering that the pumped deep-sea water is as cold as 2.3 ¢J on average, 15 ¢J is also attractive to minimize the energy cost for heating.
Continuous cultivation
At the beginning, Ch. Ceratosporum was inoculated at the density of 104cells/ml, and cultured under batch conditions for two days. Then the continuous stage was started by adding deep-sea water after the cell density reached 10¡Ñ104 cells/ml. In continuous stage, the turnover rate of deep-sea water was elevated step by step from 0.2/day to 1.2/day (Fig.3). As the result, the cell density fluctuated from 10¡Ñ104 to 61¡Ñ104cells/ml. When the turnover rate was elevated, cell density decreased temporarily every time but it recovered in 10 days.
¡@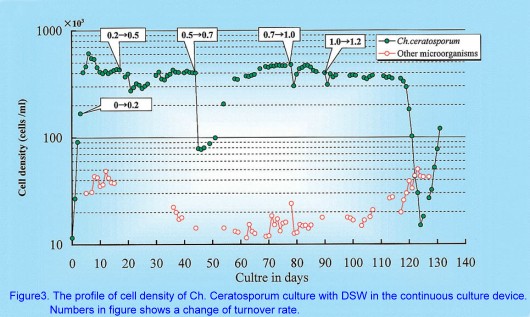
At turnover rates of 0.2/day, average cell density was 45¡Ñ104cells/ml. Then it was 36.5¡Ñ104 cells/ml at 0.5, 43.7¡Ñ104 cells/ml at 0.7, 43.7¡Ñ104 cells/ml at 1.0, 36.6¡Ñ104 cells/ml at 1.2. Average cell density was highest at a turnover rate of 0.2/day and lowest at a rate of 0.5/day(Fig 4). No trends were seen between turnover rate and cell density. On the contrary, the yield of cells for one day increased in proportion to turnover rates from 0.2 to 1.0, but saturated at the turnover rate of 1.0. The continuous culture was efficient at turnover rates from 1.0 to 1.2/day in this system. In this way, a high level of cell density was obtained for as long as 125 days in continuous culture. At the turnover rate of 1.0/day, although microalgae and protozoans invaded and increased up to 5¡Ñ104 cells/ml, Ch. Ceratosporum kept its dominance. But at the latter of turnover rate 1.2/day, other microorganisms increased about 10¡Ñ104 cells/ml in the device and this shifted the balance between growth rate of Ch. Ceratosporum and turnover rate. Since contamination of other microorganisms increased with the increase in turnover rate, this continuous cultivation system should be driven with turnover rate less than 1.2/day.
¡@
Nitrate and phosphate in DSW were almost consumed. In contrast, the consumption rate of silicate was lower than nitrate and phosphate and decreased with turnover rate although more than 80% of the silicate was consumed. We can say that Ch.Ceratosporum could efficiently consume the nutrients of DSW in the continuous culture device. The long-term continuous culture of a planktonic diatom Ch. ceratosporum was established by using DSW as the sole source of nutrients in a chemostat system.
In our institute, yield of Ch. Ceratosporum was fed to planktonic larvae of a red sea urchin Pseudocentrostus depresuss as live food (Tenjin and Ishii 1984), and we produced young sea urchins. The yield of Ch. Ceratosporum may support a new project in the multiple/cascade use of deep-sea water .
REFERENCES

FUKAMI, K., NISHIJIMA, T. and HATA, Y. (1992). Availability of deep seawater and effects of bacteria isolated from deep seawater on the mass culture of food micoroalga Chaetoceros ceratosporum. Bull. Japan. Soc. Fish., 58 (5), 931-936.

FUKAMI, K., KAWAI, A., OKABE, M., HOTTA, T., MORIYAMA, T., DOI, S., NISHIJIMA, T. YAMAGUTI, M. and TANIGUCHI, M. (1998). Continuous and simultaneous cultivation of benthic food diatom Nitzschia sp. and abalone Haliotis sieboldii by using deep seawater. J. Mar. Biotec., 6, 237-240.

MATUBAYASHI, T., MURAYAMA, I., KIDO, S., ANDO, Y., NAKASHIMA, T. and TOYOTA, T. (1994). Effects of deep seawater on the growth of several species of marine micro-algae. J. Applied Phycology, 6, 75-77.

MATSUZATO, H. (1998). Utilization study of deep-sea water in Toyama Prefecture. Marine coastal environment, 1266-1279, Fuji-technosystem. Co. Ltd., Tokyo (in Japanese).

NAKAMURA, K. (1999). Utilization study of deep-sea water in Toyama Prefecture. JADOWA News. 1, 2, 5-8 (in Japanese).

NAKASHIMA, T. (1988). Effects of deep sea water on the growth of marine diatom species Skeletlnema costatum. Bull. Plankton Soc.Japan, 35, 45-55 (in Japanese with English abstract).

NAKASHIMA, T. (1992 a). Factor liberating growth lag of a diatom, Skeletlnema costatum, in deepsea water ¢¹.Liberation by adding various organic matter. Bull. Plankton Soc.Japan, 38, 93-104 (in Japanese with English abstract).

NAKASHIMA, T. (1992 b). Factor liberating growth lag of a diatom, Skeletlnema costatum, in deepsea water ¢º .liberation by mixing with surface sea water. Bull. Plankton Soc.Japan, 38, 105-111 (in Japanese with English abstract).

NAKASHIMA, T. (1995). Research activities at Kochi artificial upwelling laboratory on the utilization of deep seawater resources. IOA Newsletter, 6, No.4/Winter. 1-5.

TANAKA, Y. (1982). The thermotolerant unicellular diatom, Chaetoceros ceratosporum OSTENFELD, as a useful feed to bivalve larvae. Bull. Natl. Res. Inst. Aquaculture, 3, 31-36 (in Japanese with English abstract).

TAKANO, H. (1967). Rearing experiments of brine shrimp on diatom diet. Bull. Tokai Reg. Fish. Res. Lab., 52, 1-11.

TENJIN, A and ISHII, T. (1984). Studies on the living food for planktonic larvae of sea urchin and bivalve. Bull. Fukushima Pref. Fish Farm. Exp. Sat., 1, 29-34 (in Japanese).

UMEDA, I. (1998). Marine coastal environment, 1326-1330, Fuji-technosystem. Co. Ltd., Tokyo (in Japanese).
To determine the temperature of continuous cultivation, batch culture was carried out for 14 days using 200 ml flasks filled with 100ml DSW at four temperatures--10, 15, 20 and 25 ¢J --and cell density was checked every day. Each flask was aerated at a rate of 100ml/min. Illumination was provided by white fluorescent tubes, giving a photo flux density of 80 £g E/m2S for 24 hours. At the beginning, 5,000 cells/ml of Ch. Ceratosporum was inoculated in each flask. Culture growth was measured by counting cell numbers with a hemacytometer.
¡@
Continuous cultivation
We used an Ebara Photo-bio-reactor (PBR-60, Ebara Co. Ltd.) which is device for phytoplankton, and cultured Ch. ceratosporum with a chemostat system in which the supply of culture medium and collection of phytoplankton yield are conducted by one roller pump (Fig. 2). The volume of the culture tank is 180 L. The pumped deep-sea water, as the culture medium, is clean; two sediment tanks were placed before pumping to minimize contamination and no filtration was used. Illumination was provided by 6 built-in halogen lamps with a photo flux density between 90 to 120 £g E/m2S for 24 hours. For water temperature control, 15 degree-C was targeted but the temperature increased up to 17±1 ¢J due to heat emission from the halogen lamps. pH was adjusted to 7.8±0.1 using carbon dioxide since the pH of deep sea water is around 7.8. Culture medium was stirred at 50 rpm and aerated at a rate of 60L/min. Cultivation was initiated under batch conditions and shifted to continuous stage by adding DSW after cell density reached 10¡Ñ104 cells/ml. The turnover rate of DSW was elevated from 0.2/day to 1.2/day gradually during continuous cultivation.
RESULTS AND DISCUSSION
Preliminary Batch cultivation
The growth curve of Ch. Ceratosporum at each batch culture is shown in Fig. 1. At 10, cell density increased slowly and peaked at 3.6¡Ñ104 cells/ml in 14 days. At 15¢J, cell density reached a peak of 14¡Ñ104 cells/ml in 7 days and then it fluctuated from 9¡Ñ104to 14¡Ñ104 cells/ml. At 20 and 25¢J, cell density reached 14¡Ñ104 and 17¡Ñ104 in 3 days, respectively, but after 6 days density decreased gradually and reached about 2¡Ñ104 cells/ml in 14 days. As the result, we selected 15 ¢J as the water temperature of continuous culture since the maximum yield was not so far from those of higher temperatures. Considering that the pumped deep-sea water is as cold as 2.3 ¢J on average, 15 ¢J is also attractive to minimize the energy cost for heating.
Continuous cultivation
At the beginning, Ch. Ceratosporum was inoculated at the density of 104cells/ml, and cultured under batch conditions for two days. Then the continuous stage was started by adding deep-sea water after the cell density reached 10¡Ñ104 cells/ml. In continuous stage, the turnover rate of deep-sea water was elevated step by step from 0.2/day to 1.2/day (Fig.3). As the result, the cell density fluctuated from 10¡Ñ104 to 61¡Ñ104cells/ml. When the turnover rate was elevated, cell density decreased temporarily every time but it recovered in 10 days.
¡@
At turnover rates of 0.2/day, average cell density was 45¡Ñ104cells/ml. Then it was 36.5¡Ñ104 cells/ml at 0.5, 43.7¡Ñ104 cells/ml at 0.7, 43.7¡Ñ104 cells/ml at 1.0, 36.6¡Ñ104 cells/ml at 1.2. Average cell density was highest at a turnover rate of 0.2/day and lowest at a rate of 0.5/day(Fig 4). No trends were seen between turnover rate and cell density. On the contrary, the yield of cells for one day increased in proportion to turnover rates from 0.2 to 1.0, but saturated at the turnover rate of 1.0. The continuous culture was efficient at turnover rates from 1.0 to 1.2/day in this system. In this way, a high level of cell density was obtained for as long as 125 days in continuous culture. At the turnover rate of 1.0/day, although microalgae and protozoans invaded and increased up to 5¡Ñ104 cells/ml, Ch. Ceratosporum kept its dominance. But at the latter of turnover rate 1.2/day, other microorganisms increased about 10¡Ñ104 cells/ml in the device and this shifted the balance between growth rate of Ch. Ceratosporum and turnover rate. Since contamination of other microorganisms increased with the increase in turnover rate, this continuous cultivation system should be driven with turnover rate less than 1.2/day.
¡@
Nitrate and phosphate in DSW were almost consumed. In contrast, the consumption rate of silicate was lower than nitrate and phosphate and decreased with turnover rate although more than 80% of the silicate was consumed. We can say that Ch.Ceratosporum could efficiently consume the nutrients of DSW in the continuous culture device. The long-term continuous culture of a planktonic diatom Ch. ceratosporum was established by using DSW as the sole source of nutrients in a chemostat system.
In our institute, yield of Ch. Ceratosporum was fed to planktonic larvae of a red sea urchin Pseudocentrostus depresuss as live food (Tenjin and Ishii 1984), and we produced young sea urchins. The yield of Ch. Ceratosporum may support a new project in the multiple/cascade use of deep-sea water .
REFERENCES
|
FUKAMI, K., NISHIJIMA, T. and HATA, Y. (1992). Availability of deep seawater and effects of bacteria isolated from deep seawater on the mass culture of food micoroalga Chaetoceros ceratosporum. Bull. Japan. Soc. Fish., 58 (5), 931-936. |
|
|
FUKAMI, K., KAWAI, A., OKABE, M., HOTTA, T., MORIYAMA, T., DOI, S., NISHIJIMA, T. YAMAGUTI, M. and TANIGUCHI, M. (1998). Continuous and simultaneous cultivation of benthic food diatom Nitzschia sp. and abalone Haliotis sieboldii by using deep seawater. J. Mar. Biotec., 6, 237-240. |
|
|
MATUBAYASHI, T., MURAYAMA, I., KIDO, S., ANDO, Y., NAKASHIMA, T. and TOYOTA, T. (1994). Effects of deep seawater on the growth of several species of marine micro-algae. J. Applied Phycology, 6, 75-77. |
|
|
MATSUZATO, H. (1998). Utilization study of deep-sea water in Toyama Prefecture. Marine coastal environment, 1266-1279, Fuji-technosystem. Co. Ltd., Tokyo (in Japanese). |
|
|
NAKAMURA, K. (1999). Utilization study of deep-sea water in Toyama Prefecture. JADOWA News. 1, 2, 5-8 (in Japanese). |
|
|
NAKASHIMA, T. (1988). Effects of deep sea water on the growth of marine diatom species Skeletlnema costatum. Bull. Plankton Soc.Japan, 35, 45-55 (in Japanese with English abstract). |
|
|
NAKASHIMA, T. (1992 a). Factor liberating growth lag of a diatom, Skeletlnema costatum, in deepsea water ¢¹.Liberation by adding various organic matter. Bull. Plankton Soc.Japan, 38, 93-104 (in Japanese with English abstract). |
|
|
NAKASHIMA, T. (1992 b). Factor liberating growth lag of a diatom, Skeletlnema costatum, in deepsea water ¢º .liberation by mixing with surface sea water. Bull. Plankton Soc.Japan, 38, 105-111 (in Japanese with English abstract). |
|
|
NAKASHIMA, T. (1995). Research activities at Kochi artificial upwelling laboratory on the utilization of deep seawater resources. IOA Newsletter, 6, No.4/Winter. 1-5. |
|
|
TANAKA, Y. (1982). The thermotolerant unicellular diatom, Chaetoceros ceratosporum OSTENFELD, as a useful feed to bivalve larvae. Bull. Natl. Res. Inst. Aquaculture, 3, 31-36 (in Japanese with English abstract). |
|
|
TAKANO, H. (1967). Rearing experiments of brine shrimp on diatom diet. Bull. Tokai Reg. Fish. Res. Lab., 52, 1-11. |
|
|
TENJIN, A and ISHII, T. (1984). Studies on the living food for planktonic larvae of sea urchin and bivalve. Bull. Fukushima Pref. Fish Farm. Exp. Sat., 1, 29-34 (in Japanese). |
|
|
UMEDA, I. (1998). Marine coastal environment, 1326-1330, Fuji-technosystem. Co. Ltd., Tokyo (in Japanese). |
